


Water Testing - pH -
Test Strips 0 - 14
1:12
Water Testing - pH -
Comparing Testing Methods
3:33
Water Testing - pH -
Home Made Litmus Paper
2:46
Phosphate
In Boilers, with adequate alkalinity, addition of sodium phosphate produces an insoluble precipitate with magnesium and calcium, which is then removed during bottom blowdowns.
High Range (Boilers)
Phosphate = PO
4
-3
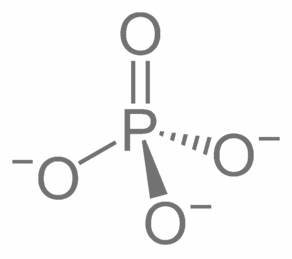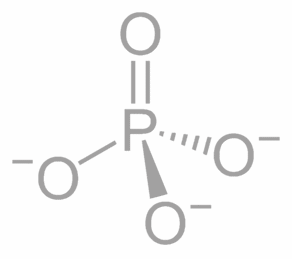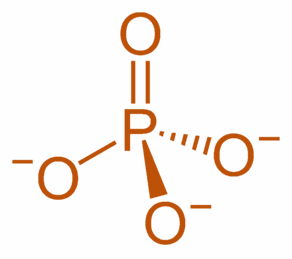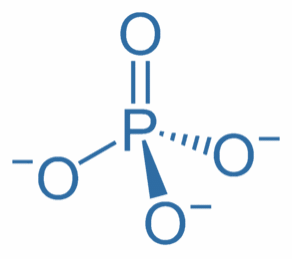
ULR: Ultra Low Range (for fish tanks)
Water Testing -
Phosphate -
Color Comparator Test
1:29
Water Testing -
Phosphate -
ULR Phosphate Colorimeter
2:23
Water Testing -
Phosphonate -
Easy Scale Removal
1:02
Treatment chemicals disrupt scale crystal formation making the scale "soft" and easy to remove, such as with a pressure washer.
Phosphonate

Not to be confused with phosphate
In cooling towers, phosphonates (PO ) are effective chelating agents with water hardness calcium and magnesium. In this way, they interfere with scale crystal formations, increasing saturation levels and also, they make scale softer, facilitating easier removal during cleaning.
3
-3
Videos
Water Testing -
Phosphonate -
Testing
11:17
Sulfite
Sodium sulfite is widely used as an oxygen scavenger in low and medium pressure boiler systems due to its comparatively low cost and non-toxicity. It can be used in either solid or liquid (sodium bisulfite dissolved in water) form. It is best added to any equipment where makeup water is to be heated, such as a DA tank.
Boiler water is analyzed for residual, unused sulfite as that this indicates there is no free oxygen in the water. Free oxygen reacts with steel and rusts, forming pit type holes in boiler tubes.
% Equivalent Replacement Chart


Videos


Sodium (Na) is just a carrier for the sulfite. Potassium (K) can easily be the carrier, albeit, with slightly different properties.
Water Testing -
Sulfite -
Hot vs Cold Sample
2:52
Water Testing -
Sulfite -
Titration Test
3:43
If the sample sits too long, it may pick up oxygen out of the atmosphere and produce inaccurate results.







